cfRNA用于非侵入性健康监测的优势
循环游离RNA(cfRNA,也称为C-RNA)是非侵入性健康评估的一个有前景的替代方案。与DNA不同,细胞的RNA转录组是动态的并具有组织特异性。cfRNA由各种组织通过细胞凋亡、微泡脱落以及外泌体信号转导等细胞过程释放到循环血中。cfRNA很稳定,其包含在囊泡内,防止被核酸酶降解。由于来源不同,cfRNA的检测结果反映了全身不同组织中基因表达、细胞间信号传导和细胞死亡程度的组织特异性变化(图1)5-9

图1. 图1.循环cfRNA可通过标准抽血获得,反映了所有身体系统的分子变化。
cfRNA文库制备和测序的优化实验方案
尽管cfRNA具有极高的信息潜力,但在实践中也确实存在挑战。每毫升血浆仅能获得几纳克cfRNA,其中包含全长RNA和RNA片段。人血液中最丰富的转录本是核糖体RNA(rRNA)和球蛋白RNA,它们会掩盖其他信息更丰富的转录本的信号。为了解决这些问题,Illumina的研究团队开发了一种适合cfRNA的低产量和部分片段化性质的工作流程。我们并未采用标准的去除方法来除去rRNA,而是使用全部cfRNA制备文库,然后针对整个人外显子组进行探针辅助富集。
cfRNA测序的优化工作流程大幅提高了外显子cfRNA信号,大大降低了低起始量样本的失败风险,并且兼容室温下过夜运输的血液样本。原始实验方案使用基于连接的文库制备来产生高质量全转录组cfRNA测序数据1。进一步的开发工作表明,基于酶切法片段化的RNA文库制备试剂盒能够为后续研究提供更高的灵敏度和通量(图2、图3)10
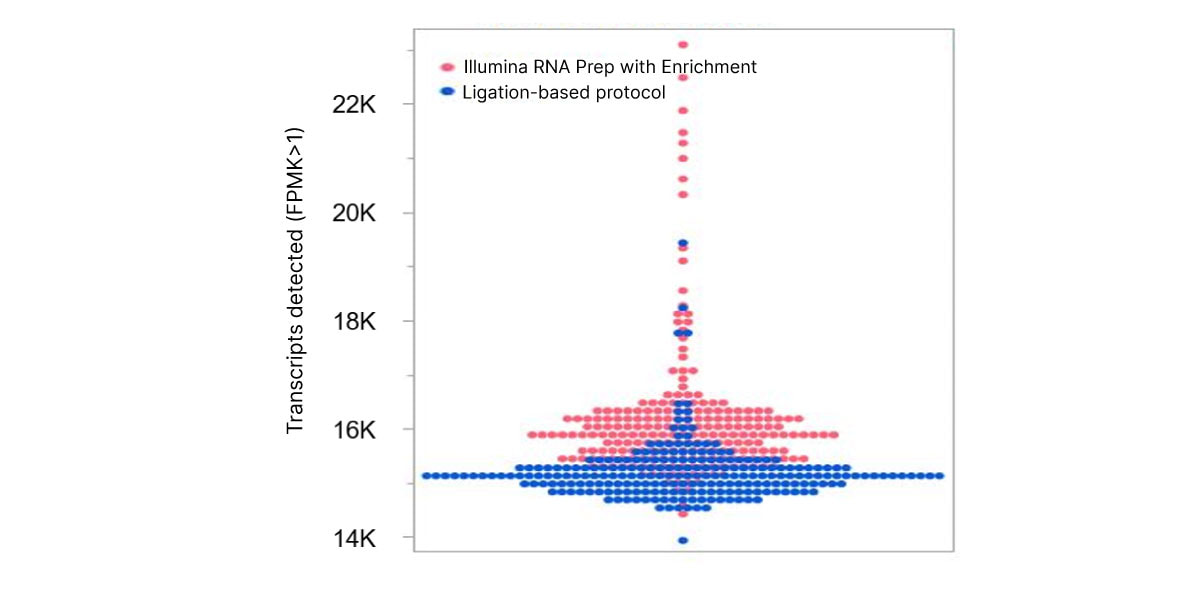
图2. Illumina RNA Prep with Enrichment可实现更高的灵敏度,与基于连接的实验方案相比,能够检测更多的循环转录本。
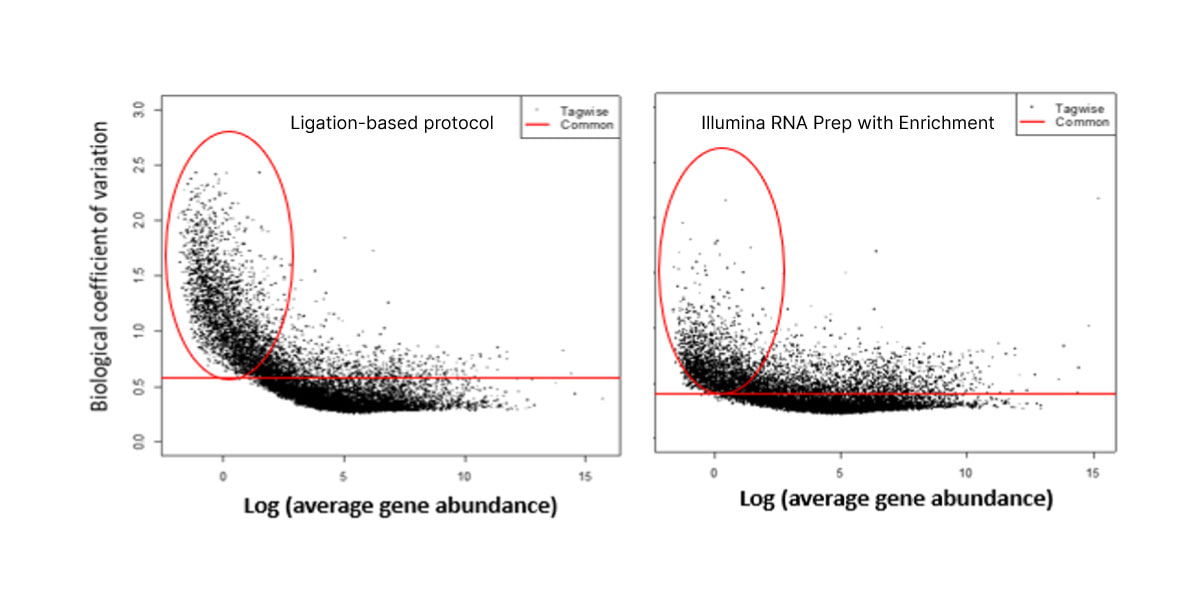
图3. 与基于连接的实验方案相比,Illumina RNA Prep with Enrichment对于低丰度cfRNA具有更低的噪声。
鉴定早发性子痫前期cfRNA特征的概念验证研究
我们最近发表在 Science Translational Medicine 的文章描述了我们如何在概念验证研究中建立和应用此工作流程,以鉴定与危险的妊娠并发症子痫前期相关的cfRNA改变1 。团队收集了113份血液样本,其中40份来自患有严重早发性子痫前期的女性,73份来自健康妊娠的女性。
在适用于在有噪声的数据中明确趋势的机器学习的帮助下,我们发现了一种cfRNA特征,它可以捕获各种身体系统机能障碍,并在不同队列中对子痫前期状态进行准确分类。我们在循环中发现了30种与无并发症妊娠相比丰度存在明显差异的转录本。这些转录本的基因本体注释和组织表达模式与子痫前期的胎盘功能障碍、胎儿发育异常以及母体免疫和心血管失调等特征一致。(表1)。
已发现的与子痫前期相关的循环转录本的要点
我们在子痫前期患者中观察到的cfRNA改变与疾病的生物学特征匹配,证实了该方法的有效性。在鉴定出的30种基因转录本中,超过一半此前已发现与子痫前期相关(表1)11-29 T。发生改变的cfRNA转录本捕获了来自多个不同器官系统的信息,代表了母体、胎盘和胎儿组织的贡献。例如,我们发现了胎盘中的功能障碍,以及母体血压调节的改变。
以下基因分类重点介绍了cfRNA捕获的受影响的生物学过程的具体实例。
IGF信号转导:影响胎儿生长和发育
子痫前期与生物可利用的胰岛素样生长因子(IGF)水平偏低相关,IGF是胎儿生长和发育的重要调控因子30-32 IGFBP5, PAPPA2, HTRA4和 PRG2都与IGF信号转导调控相关,并且发现它们在早发性子痫前期患者中有所升高。
妊娠期:早产风险
CRH, ZEB1, and PNMT(与妊娠期和分娩发动相关的基因33-35 )在子痫前期cfRNA中表达上调。早产是早发性子痫前期的常见结果,本研究中几乎所有受影响的参与者都出现早产。
血管生成:胎盘发育受损
本研究中鉴定出的十种转录本对于调控血管生成十分重要:APOLD1, LEP, SEMA3G, ADAMTS1, TIMP3, ADAMTS2, HTRA4, HSPA12B, SLC9A3R2, 和TIMP4。血管生成是整个妊娠过程中胎盘正常发育的重要部分,但在子痫前期妊娠中会受到影响。36,37
血压调节:高血压
高血压是子痫前期的典型症状。与血压调节相关的多个转录本, ALOX15B, PNMT, ARHGEF25, APOLD1和 CRH,在该队列中显示cfRNA转录本水平升高。
树突状细胞活性:影响妊娠免疫耐受
在子痫前期患者中,免疫系统树突状细胞标志物也显示出异常表达:CLEC4C, PLD4, TIMP3和 VSIG4。树突状细胞在免疫系统对妊娠期胎盘和胎儿的耐受性方面发挥着关键作用,并被认为是子痫前期的潜在驱动因素。38-40
| 基因名称 | 子痫前期中的倍数变化 | 蛋白功能 |
|---|---|---|
胎儿组织表达 |
||
IGFBP5* |
+3.6 |
IGF信号转导 |
ALOX15B* |
+5.7 |
血压调节 |
NES* |
+4.5 |
胎儿发育 |
TEAD4 |
+3.3 |
细胞增殖 |
PITPNM3 |
+3.2 |
细胞增殖 |
CUX2 |
-3.3 |
细胞增殖 |
FAM107A |
+5.0 |
细胞增殖和迁移 |
PRX* |
+3.8 |
细胞结构 |
AMPH |
+5.0 |
内吞作用 |
胎盘组织表达 |
||
PAPPA2* |
+4.9 |
IGF 信号转导 |
HTRA4* |
+3.9 |
IGF 信号转导;血管生成 |
LEP* |
+10.7 |
血管生成 |
SEMA3G |
+3.5 |
血管生成 |
APOLD1* |
+3.4 |
血管生成,血压调节 |
VSIG4* |
+8.1 |
树突状细胞活性 |
胎盘/胎儿组织表达 |
||
PRG2* |
+5.2 |
IGF 信号转导 |
CRH* |
+5.7 |
妊娠期;血压调节 |
ADAMTS1* |
+3.5 |
血管生成 |
ADAMTS2 |
+11.6 |
血管生成 |
TIMP3* |
+4.1 |
血管生成;树突状细胞活性 |
其他组织表达 |
||
PNMT |
+3.8 |
妊娠期;血压调节 |
ZEB1* |
+2.8 |
妊娠期;血压调节 |
TIMP4* |
+4.3 |
血管生成 |
SLC9A3R2 |
+3.6 |
血管生成 |
HSPA12B |
+3.5 |
血管生成 |
ARHGEF25 |
+4.1 |
血压调节 |
PLD4 |
-3.0 |
树突状细胞活性 |
CLEC4C* |
-3.6 |
树突状细胞活性 |
DAAM2* |
+5.6 |
细胞增殖 |
KRT5* |
-5.8 |
细胞结构 |
* 此前已发现与子痫前期相关的基因11-29
复杂疾病的非侵入性生物标志物发现和诊断
我们的研究表明,cfRNA测序可以检测血液中子痫前期的分子信号,实时监测母体、胎儿和胎盘功能。此研究是概念验证研究,证明血液中存在稳定的疾病特征。样本在诊断时从患者收集,意味着症状已经出现。但是,指示子痫前期的cfRNA转录本在出现症状前可能就已经存在,需要进一步的研究来确定预测性生物标志物发现的潜力。这一检测模式和在妊娠前三个月进行子痫前期筛查有可能发现具有更高妊娠并发症风险的孕妇,并实现早期干预。41
本研究强调了cfRNA作为研究和生物标志物发现工具,对健康进行非侵入性全面分子监测的广泛潜力。cfRNA测序的优化实验方案可实现循环转录本的可靠检测,以识别与疾病相关的可重复的生物学相关改变。许多研究人员正在评估循环RNA特征作为可获得的独特生物标志物,用于诊断和监测阿茨海默病、癌症和其他复杂疾病的实用性。42-46 这一技术的进一步应用为改变我们对许多疾病的理解、诊断和治疗提供了可能。
了解更多
应用说明:改善对循环转录本的检测性能
参考文献
- Munchel S, Rohrback S, Randise-Hinchliff C, et al. Circulating transcripts in maternal blood reflect a molecular signature of early-onset preeclampsia. Sci Transl Med.2020;12(550):eaaz0131. doi:10.1126/scitranslmed.aaz0131
- Parikh AR, Leshchiner I, Elagina L, et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat Med.2019;25(9):1415-1421. doi:10.1038/s41591-019-0561-9
- Leighl NB, Page RD, Raymond VM, et al. Clinical Utility of Comprehensive Cell free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic Non small Cell Lung Cancer. Clin Cancer Res.2019;25(15):4691 4700. doi:10.1158/1078 0432.CCR 19 062z
- Palmero R, Taus A, Viteri S, et al. Biomarker Discovery and Outcomes for Comprehensive Cell Free Circulating Tumor DNA Versus Standdard of Care Tissue Testing in Advanced Non Small Cell Lung Cancer. JCO Precision Oncology.2021;5:93 102. doi:10.1200/PO.20.00241
- Turchinovich A, Drapkina O, Tonevitsky A. Transcriptome of extracellular vesicles: State-of-the-art. Front Immunol.2019;10:202. doi:10.3389/fimmu.2019.00202
- Anfossi S, Babayan A, Pantel K, Calin G. Clinical utility of circulating noncoding RNAs—an update. Nat Rev Clin Oncol.2018;15:541-563.
- Li Y, Elashoff D, Oh M, et al. Serum circulating human mRNA profiling and its utility for oral cancer detection. J Clin Oncol.2006;24:1754-1760. doi:10.1200/JCO.2005.03.7598
- Starling S. A molecular signal for preeclampsia. Nat Rev Endocrinol.2020;16:471. doi:10.1038/s41574-020-0397-x
- Lo Y, Ng K, Tsui B, Chiu W, inventors; Chinese University of Hong Kong, assignee. Circulating mRNA as diagnostic markers. US patent application 20040203037A1. October 14, 2004.
- Illumina. Improved detection of circulating transcripts. Accessed November 19, 2021.
- Jia Y, Li T, Huang X, et al. Dysregulated DNA Methyltransferase 3A Upregulates IGFBP5 to Suppress Trophoblast Cell Migration and Invasion in Preeclampsia. Hypertension.2017;69(2):356-366. doi:10.1161/HYPERTENSIONAHA.116.08483
- Wang Y, Zhu D, An Y, Sun J, Cai L, Zheng J. Preeclampsia activates 15-lipoxygenase and its metabolite 15-hydroxyeicosatetraenoic acid enhances constriction in umbilical arteries. Prostaglandins Leukot Essent Fatty Acids.2012;86(1-2):79-84. doi:10.1016/j.plefa.2011.10.006
- Hwang HS, Cho NH, Maeng YS, Kang MH, Park YW, Kim YH. Differential expression of nestin in normal and pre-eclamptic human placentas. Acta Obstet Gynecol Scand.2007;86(8):909-914. doi:10.1080/00016340701417018
- Tan KH, Tan SS, Sze SK, Lee WK, Ng MJ, Lim SK. Plasma biomarker discovery in preeclampsia using a novel differential isolation technology for circulating extracellular vesicles. Am J Obstet Gynecol.2014;211(4):380.e1-380.e3813. doi:10.1016/j.ajog.2014.03.038
- Kramer AW, Lamale-Smith LM, Winn VD. Differential expression of human placental PAPP-A2 over gestation and in preeclampsia. Placenta.2016;37:19-25. doi:10.1016/j.placenta.2015.11.004
- Singh H, Zhao M, Chen Q, et al. Human HtrA4 Expression Is Restricted to the Placenta, Is Significantly Up-Regulated in Early-Onset Preeclampsia, and High Levels of HtrA4 Cause Endothelial Dysfunction. J Clin Endocrinol Metab.2015;100(7):E936-E945. doi:10.1210/jc.2014-3969
- Xiang Y, Cheng Y, Li X, et al. Up-regulated expression and aberrant DNA methylation of LEP and SH3PXD2A in pre-eclampsia. PLoS One.2013;8(3):e59753. doi:10.1371/journal.pone.0059753
- Junus K, Centlow M, Wikström AK, Larsson I, Hansson SR, Olovsson M. Gene expression profiling of placentae from women with early- and late-onset pre-eclampsia: down-regulation of the angiogenesis-related genes ACVRL1 and EGFL7 in early-onset disease. Mol Hum Reprod.2012;18(3):146-155. doi:10.1093/molehr/gar067
- Textoris J, Ivorra D, Ben Amara A, et al. Evaluation of current and new biomarkers in severe preeclampsia: a microarray approach reveals the VSIG4 gene as a potential blood biomarker. PLoS One.2013;8(12):e82638. doi:10.1371/journal.pone.0082638
- Gormley M, Ona K, Kapidzic M, Garrido-Gomez T, Zdravkovic T, Fisher SJ. Preeclampsia: novel insights from global RNA profiling of trophoblast subpopulations. Am J Obstet Gynecol.2017;217(2):200.e1-200.e17. doi:10.1016/j.ajog.2017.03.017
- Ng EK, Leung TN, Tsui NB, et al. The concentration of circulating corticotropin-releasing hormone mRNA in maternal plasma is increased in preeclampsia. Clin Chem.2003;49(5):727-731. doi:10.1373/49.5.727
- Purwosunu Y, Sekizawa A, Farina A, et al. Cell-free mRNA concentrations of CRH, PLAC1, and selectin-P are increased in the plasma of pregnant women with preeclampsia. Prenat Diagn.2007;27(8):772-777. doi:10.1002/pd.1780
- Namlı Kalem M, Kalem Z, Yüce T, Soylemez F. ADAMTS 1, 4, 12, and 13 levels in maternal blood, cord blood, and placenta in preeclampsia. Hypertens Pregnancy.2018;37(1):9-17. doi:10.1080/10641955.2017.1397690
- Xie D, Zhu J, Liu Q, et al. Dysregulation of HDAC9 Represses Trophoblast Cell Migration and Invasion Through TIMP3 Activation in Preeclampsia. Am J Hypertens.2019;32(5):515-523. doi:10.1093/ajh/hpz006
- Yang X, Meng T. MicroRNA-431 affects trophoblast migration and invasion by targeting ZEB1 in preeclampsia. Gene.2019;683:225-232. doi:10.1016/j.gene.2018.10.015
- Sandrim VC, Diniz S, Eleuterio NM, Gomes KB, Dusse LMS, Cavalli RC. Higher levels of circulating TIMP-4 in preeclampsia is strongly associated with clinical parameters and microRNA. Clin Exp Hypertens.2018;40(7):609-612. doi:10.1080/10641963.2017.1411499
- Darmochwal-Kolarz D, Rolinski J, Tabarkiewicz J, et al. Myeloid and lymphoid dendritic cells in normal pregnancy and pre-eclampsia. Clin Exp Immunol.2003;132(2):339-344. doi:10.1046/j.1365-2249.2003.02136.x
- Løset M, Mundal SB, Johnson MP, et al. A transcriptional profile of the decidua in preeclampsia. Am J Obstet Gynecol.2011;204(1):84.e1-84.e27. doi:10.1016/j.ajog.2010.08.043
- Tan KH, Tan SS, Sze SK, Lee WK, Ng MJ, Lim SK. Plasma biomarker discovery in preeclampsia using a novel differential isolation technology for circulating extracellular vesicles. Am J Obstet Gynecol.2014;211(4):380.e1-380.e3813. doi:10.1016/j.ajog.2014.03.038
- Vatten LJ, Nilsen TI, Juul A, Jeansson S, Jenum PA, Eskild A. Changes in circulating level of IGF-I and IGF-binding protein-1 from the first to second trimester as predictors of preeclampsia. Eur J Endocrinol.2008;158(1):101-105. doi:10.1530/EJE-07-0386
- Halhali A, Tovar AR, Torres N, Bourges H, Garabedian M, Larrea F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J Clin Endocrinol Metab.2000;85(5):1828-1833. doi:10.1210/jcem.85.5.6528
- Kappou D, Vrachnis N, Sifakis S. Recent Insights into the Role of the Insulin-Like Growth Factor Axis in Preeclampsia. In: Sifakis S, ed. From Preconception to Postpartum.InTech; 2012:147-160.
- Xu YJ, Ren LD, Zhai SS, et al. OXTR and ZEB1 expression before and after progesterone dosing in pregnant women with threatened premature labor. Eur Rev Med Pharmacol Sci.2017;21(14):3164-3168.
- Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci U S A.2010;107(48):20828-20833. doi:10.1073/pnas.1008301107
- Smith R, Nicholson RC. Corticotrophin releasing hormone and the timing of birth. Front Biosci.2007;12:912-918. doi:10.2741/2113
- Cerdeira AS, Karumanchi SA. Angiogenic factors in preeclampsia and related disorders. Cold Spring Harb Perspect Med.2012;2(11):a006585. doi:10.1101/cshperspect.a006585
- Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol.2011;31(1):33-46. doi:10.1016/j.semnephrol.2010.10.004
- Li J, Huang L, Wang S, Zhang Z. The prevalence of regulatory T and dendritic cells is altered in peripheral blood of women with pre-eclampsia. Pregnancy Hypertens.2019;17:233-240. doi:10.1016/j.preghy.2019.07.003
- Huang SJ, Chen CP, Schatz F, Rahman M, Abrahams VM, Lockwood CJ. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol.2008;214(3):328-336. doi:10.1002/path.2257
- Wang J, Tao YM, Cheng XY, et al. Dendritic cells derived from preeclampsia patients influence Th1/Th17 cell differentiation in vitro. Int J Clin Exp Med.2014;7(12):5303-5309.
- Rasmussen M, Reddy M, Nolan R, et al. RNA profiles reveal signatures of future health and disease in pregnancy. Nature.2022;10.1038/s41586-021-04249-w. doi:10.1038/s41586-021-04249-w
- Metzenmacher M, Váraljai R, Hegedüs B, et al. Plasma Next Generation Sequencing and Droplet Digital-qPCR-Based Quantification of Circulating Cell-Free RNA for Noninvasive Early Detection of Cancer. Cancers (Basel).2020;12(2):353. doi:10.3390/cancers12020353
- Ibarra A, Zhuang J, Zhao Y, et al. Non-invasive characterization of human bone marrow stimulation and reconstitution by cell-free messenger RNA sequencing. Nat Commun.2020;11(1):400. doi:10.1038/s41467-019-14253-4
- Toden S, Zhuang J, Acosta AD, et al. Noninvasive characterization of Alzheimer's disease by circulating, cell-free messenger RNA next-generation sequencing. Sci Adv.2020;6(50):eabb1654. doi:10.1126/sciadv.abb1654
- Hulstaert E, Morlion A, Avila Cobos F, et al. Charting Extracellular Transcriptomes in The Human Biofluid RNA Atlas. Cell Rep.2020;33(13):108552. doi:10.1016/j.celrep.2020.108552
- Larson MH, Pan W, Kim HJ, et al. A comprehensive characterization of the cell-free transcriptome reveals tissue- and subtype-specific biomarkers for cancer detection. Nat Commun.2021;12(1):2357. doi:10.1038/s41467-021-22444-1